In food, taste and aroma can be compared and contrasted in many ways. One of these examples is how they both are distinctly perceived by different specialized sensory organs.
Taste is detected by the tongue, and is a bit restricted; flavors can be basic modalities like sweet, sour, bitter, salty, and umami, or whatever lies in between. This is because the compounds in food are largely nonvolatile, meaning they don't evaporate or change state easily. This stability ensures consistent taste experiences
Aroma, on the other hand, is a bit more complex. Aroma compounds are volatile as hell, which means they evaporate easily, allowing them to rise from the food and interact with our olfactory receptors in our noses. I wanted to look deeper in the world of scent, partially because I’ve been convinced of its ability to make food taste better.
Let’s begin.
Everything starts with chemical structure
Every food contains a myriad of particles, some of which surpass others in presence and/or significance. For aroma, the more ‘important’ of these particles sometimes are called odor-active compounds. This is because they meet some odor threshold - they give off scent - and are therefore recognized as odor-active compounds.
But what determines the distribution of these compounds? Why, for instance, does an apple exude a fruity aroma rather than something entirely different?
Well, the answer is a bunch of reasons. Minute distinctions — from where the ingredients are sourced, to local environmental conditions, to even soil quality — can significantly influence the composition of volatile compounds, so, in turn, we can witness a bunch of varied aromatic outcomes within the same species or category of food.
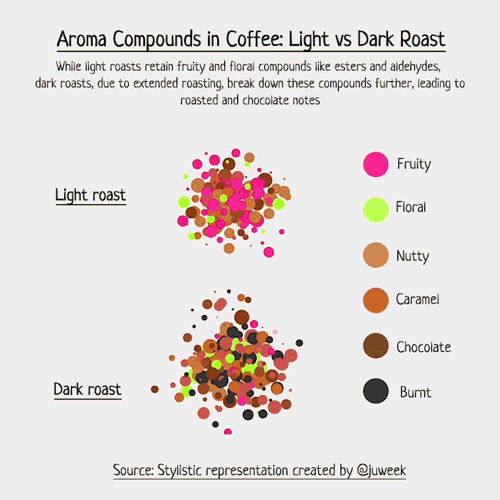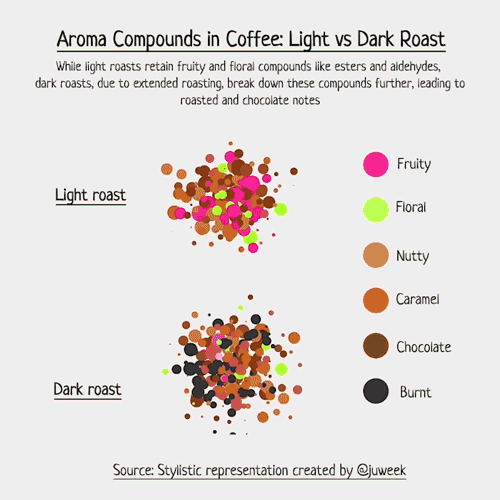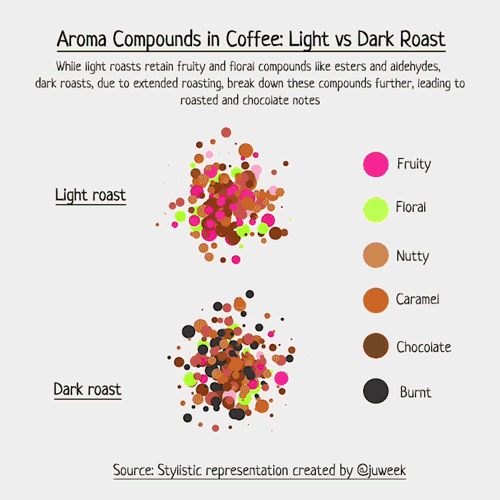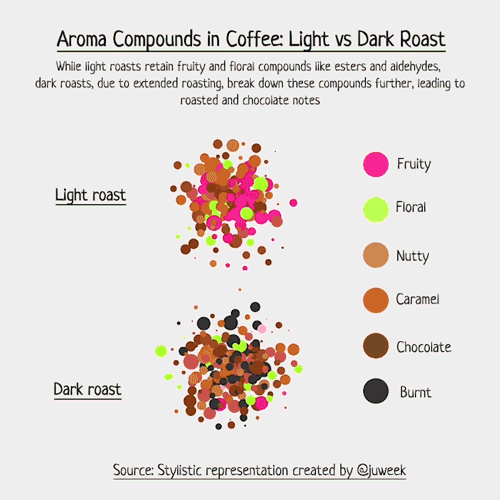
Below is an example of light roasted coffee. Compounds like Ester, Aldehyde, and Pyrazine are examples of odor-level compounds that interact within the scent profile of coffee beans, each known for giving their own scents.
But there’s something that might be even more important, and that’s how these compounds actually interact with each other. The chemical makeup of the food is only one part of the equation.
Volatile Compound Dynamics
Understanding aroma is understanding volatile compounds, and the interactions they have with each other. Like I mentioned, aroma compounds are volatile as hell, which means they clash and change and evaporate more often than taste particles, and are a lot less stable.
To make this more real, I decided to draft up examples of light vs dark roasted coffee.
Coffee roasting is a process that transforms the chemical and physical properties of green coffee beans into roasted coffee products. The roasting process is what produces the characteristic flavor - and, in part, aroma - of coffee by causing the beans to change in chemical structure.
Now, I must emphasize that this is just a stylistic visualization. Complex foods, such as coffee, can contain an array of volatile compounds, with numbers sometimes exceeding 800 (somewhere around 850, to be more specific). Charting that would be hard….
But, with my simpler examples, we can see which compounds have natural aromas, and how they interact with each other. Some combine, some overwhelm, and some just live in the same space together. The resulting ‘smell’ of coffee is a result of these compounds and their interactions with each other.
Notice now, in the dark roasted exampled, new colored chemicals may form because of the extended roasting time. Acrylamide is a good example; it smells ‘burnt’ and is something that is more likely found in darker roasts than lighter ones, hence why you see so many ‘black’ aroma particles.
For posterity’s sake, here is a summary of the aroma profiles of light, medium, and dark roasts.
Light Roasts: These are roasted for a shorter duration, which doesn’t break down as many of the coffee bean’s original volatile compounds. As a result, light roasts often maintain more of the bean's inherent flavors, leading them to exhibit fruitier and more acidic aromas.
Medium Roasts: These occupy a middle ground in roasting duration. They possess a balanced aroma, losing some of the bean's original fruity notes but not as extensively caramelized or toasted as dark roasts.
Dark Roasts: With a longer roasting duration, the intense heat breaks down many of the original fruity compounds in the beans. This results in the emergence of more caramelized, toasted, and sometimes even burnt aromatic compounds, giving the coffee a bolder and smokier profile.
Conclusion
Aroma perception isn't as simple as being the sum of individual compounds. Compounds can combine to create a greater sum than their parts, or work antagonistically where they mask each other.
Currently, we can’t really discern these aromatic profiles ourselves, so I hope this visualization is a good first step. While it is hard for me to create an actual aroma simulation that’s more accurate (at least, for now), just looking at these diagrams help me out a lot in terms of speaking and thinking critically about food.
Plus, the graphs look cool, don’t they?